Supervised ML/AI Breast Cancer Diagnostics - The Power of HealthTech
Breast cancer (BC) is the
uncontrollable growth of malignant cells in the breasts [1]. BC is the most common cancer with the
highest mortality rate. The exact cause
of breast cancer is unknown, but some women have a higher risk than others.
This includes women with a personal or family history of breast cancer and
women with certain gene mutations. Since cancer cells can metastasize, or
spread to other parts of the body, it’s important to recognize the symptoms of BC
early on. The sooner you receive a BC Diagnosis
(BCD) and start treatment, the better your outlook [2].
Conventional BCD involves imaging tests to look for BC spread. Imaging tests use x-rays, magnetic fields, sound waves, or
radioactive substances to create pictures of the inside of your body. Imaging
tests might be done for a number of reasons including [2]:
- To look at suspicious areas that might be
cancer
- To learn how far cancer might have spread
- To help determine if treatment is working
- To look for possible signs of cancer
coming back after treatment.
Despite all of
these tests, providing accurate and accessible
diagnoses remains a fundamental challenge for BCD. In the US alone
an estimated 5% of outpatients receive the wrong diagnosis every year; these
errors are particularly common when diagnosing patients with serious medical
conditions, with an estimated 20% of these patients being misdiagnosed at the
level of primary care and one in three of these misdiagnoses resulting in
serious patient harm [3].
Solution
In fact, a large
amount of data is currently available to clinicians, ranging from details of
clinical symptoms to various types of biochemical assays and outputs of imaging
devices such as chest x-ray, CT/MRI/PET/bone scans, ultrasound, etc. The study
of relevant factors and different types of large datasets jointly offers the
attractive opportunity to diagnose BC more effectively, where there are multiple possible causes of patient symptoms.
Recently, ML techniques have been successfully applied to BCD by providing an unprecedented opportunity to derive clinical insights from large-scale analysis of patient data [3, 4]. Clinical decisions have traditionally been guided by medical guidelines and accumulated experience. In contrast, ML methods add rigor to this process; algorithms can generate individualized predictions by synthesizing data across broad patient bases. Some benefits of leveraging ML in BCD are as follows [4]:
·
Find
risk factor (which features are most sensitive to BC)
·
Increase
BCD efficiency(early and accurate BCD)
·
Reduce
unnecessary hospital visits (only if needed).
The projections for this technology's growth in
the next five years are promising, as well — in fact, researchers project
a 45% growth rate
for medical AI [5].
Figure 1 illustrates the most basic application
of ML/AI in BCD as the binary classification problem. Classification
usually refers to any kind of problem where a specific type of class label is
the result to be predicted from the given input field of data. This is a task which assigns a label value (“benign” or
“malignant”) to a specific class and then can identify a particular type to be
of one kind or another. Generally, one is considered as the normal state
and the other is considered to be the abnormal state. In
BCD, ” No cancer detected” is a normal state and ” Cancer detected” represents
the abnormal state. For any model, you will require a training dataset with many examples of
inputs and outputs from which the model will train itself. The training data
must include all the possible scenarios of the problem and must have sufficient
data for each label for the model to be trained correctly. Class labels are
often returned as string values and hence needs to be encoded into an integer
like either representing 0 for “benign” or 1 for “malignant”. For each training
example, one can also create a model which predicts the Bernoulli probability
for the output. In short, it returns a discrete value [0-1] that covers all
cases and will give the output as either the outcome will have a value of 1 or
0.
SVM Algorithm
So how do we choose the best line or in general the best hyperplane that
segregates our data points? The Bias Area in Figure 2 contains outliers of both
binary classes. The SVM algorithm is robust to outliers in that it ignores
these outliers and finds the best hyperplane or decision boundary (dashed line
in Figure 2) that maximizes the margin. Specifically, SVM tries to minimize the
cost function 1/margin + penalty (aka hinge loss) representing the bias area in
Figure 2.
In
Figure 2, we have only 2 features – the age and the size of the tumor. How
about a large number of features (clump thickness, uniformity of cell
size/shape, fractal dimension, etc.)? Fortunately, SVM is effective in high dimensional cases when
N>>1. It is memory efficient as
it uses a subset of training points in the decision function called support
vectors. In addition, different kernel functions can be specified for the
decision functions and it is possible to specify custom kernels [14].
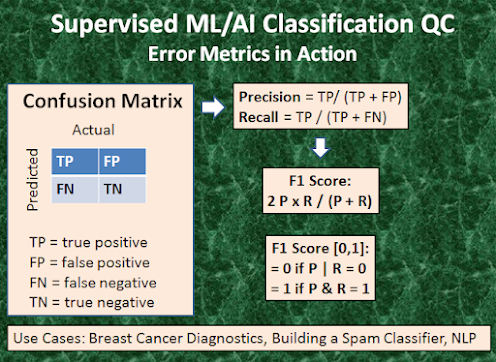
Error
Analysis
·
The target variable has two values, Positive or Negative;
·
The columns represent
the actual values of the target variable;
·
The rows represent
the predicted values of the target variable.
True Positive (TP)
·
The predicted value matches the actual value;
·
The actual value was positive and the
model predicted a positive value;
True Negative (TN)
·
The predicted value matches the actual
value;
·
The actual value was negative and the
model predicted a negative value;
False Positive (FP) aka Type 1 Error
·
The predicted value was falsely
predicted;
·
The actual value was negative but the
model predicted a positive value;
False Negative (FN) aka Type 2 Error
·
The predicted value was falsely
predicted;
·
The actual value was positive but the
model predicted a negative value.
The overall performance of the
ML algorithm can be assessed by means of the following fundamental QC metrics
(Figure 3):
Precision (P) = TP/ (TP+FP),
Recall (R ) = TP/ (TP+FN),
Accuracy =
(TP+TN)/(TP+FP+TN+FN),
F1 Score = 2*P*R/(P+R).
Precision would determine whether our model is reliable or not: of all
patients where we predicted y=1, what fraction actually has BC? Recall tells us
how many of the actual positive cases we were able to predict correctly with our
model: of all patients that actually have BC, what fraction did we correctly
detect as having BC? Accuracy 99% correct BCD means that you got 1% error on
test BC set. Finally, the F1 Score [0,1] is useful when we combine
precision/recall numbers while benchmarking different classification
algorithms, for example. Here, the objective is to resolve the precision-recall
trade-off by finding an optimal threshold TH: predict y=1 if cost function >
TH. The optimal TH finds a balance between the following two goals: suppose we
want to predict y=1 BC only if very confident (high P and low R); suppose we
want to avoid missing too many cases of BC or avoid FN (high R and low P). P is a useful
metric in cases where FP is a higher concern than FN. is important in music or
video recommendation systems, e-commerce websites, etc. Wrong results could
lead to customer churn and be harmful to the business. R is a useful metric in
cases where FN trumps FP. Recall is important in medical cases (especially in
BCD) where it doesn’t matter whether we raise a false alarm but the actual
positive cases should not go undetected! In disease screening cases, R would be
a better metric because we don’t want to accidentally discharge an infected
person and let them mix with the healthy population thereby spreading the
contagious virus. Now it is clear why Accuracy alone can be a bad metric for
our training model. But there will be cases where there is no clear distinction
between whether Precision is more important or Recall. What should we do in
those cases? We combine them! In practice, when we try to increase the
precision of our model, the recall goes down, and vice-versa. The F1-score
captures both the trends in a single value [0,1] which is the harmonic mean of
P and R values. It is maximum when P=R.
Bottom Line: In practice, we use F1-score in
combination with other evaluation metrics mentioned above which gives us a
complete picture of the result.
Python
Use Case
In this ML/AI
BCD Python open-source project [16-24] we are going to analyze and classify BC using
the public-domain BC dataset [15]. The
dataset used in this story is publicly available and was created by Dr. William
H. Wolberg, physician at the University Of Wisconsin Hospital at Madison,
Wisconsin, USA. To create the dataset Dr. Wolberg used fluid samples, taken
from patients with solid breast masses and an easy-to-use graphical computer
program called Xcyt, which is capable of perform the analysis of cytological
features based on a digital scan. The program uses a curve-fitting algorithm,
to compute ten features from each one of the cells in the sample, than it
calculates the mean value, extreme value and standard error of each feature for
the image, returning a 30 real-valued vector computed for each cell
nucleus:
1. radius (mean of distances from center to points
on the perimeter)
2. texture (standard deviation of gray-scale
values)
3.
perimeter
4.
area
5. smoothness (local variation in radius lengths)
6.
compactness (perimeter² / area — 1.0)
7. concavity (severity of concave portions of the
contour)
8. concave points (number of concave portions of the
contour)
9.
symmetry
10.
fractal dimension (“coastline
approximation” — 1)
The mean, standard error and “worst” or largest (mean
of the three largest values) of these features were computed for each image,
resulting in 30 features. For instance, field 3 is Mean Radius, field 13 is
Radius SE, field 23 is Worst Radius.
Our objective
is to create a model which will correctly classify whether the BC is of
malignant or benign type. So with the help of the supervised ML ETL pipeline in
Figure 4 if we can classify the patient having which type of BC, then it will
be easy for doctors to provide timely treatment to patients and improve the
chance of survival. The main steps of this project are outlined in Figure 4:
input data preparation, editing and splitting, Exploratory Data Analysis (EDA),
model training, testing, tuning, deployment, validation and inference. There
are no missing or null data points of the
data set.
The simplest
Python ETL pipeline is based on the scikit-learn library [6] available in the latest
release of Anaconda IDE [7]. The corresponding Jupyter notebook Python3
sequence implements the binary classification ML algorithm as follows:
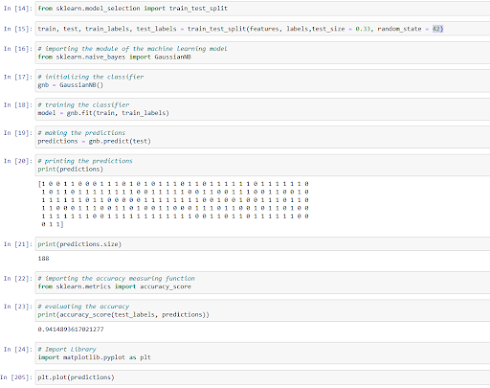
Lines 1-2
import sklearn and load_breast_cancer dataset;
Lines 3-4
store the data in a variable while creating features set and labels;
Lines 8, 9
the features type is the Numpy array;
Line 11
features size is 17070 = 569 rows x 30 columns
Line 12
feature names = 30
Line 13 view
30 features
Lines 14-15 split the data into training and
test datasets (67 and 33%, respectively) by importing the function
train_test_split from sklearn library;
Lines 17-18 let’s select the simplest Naive Bayes
algorithm that usually performs well in binary classification tasks; firstly,
import the GaussianNB module and initialize it using the GaussianNB() function;
then train the model by fitting it to the data in the dataset using the fit()
method;
Lines 19-21 view the predictions as 0 and 1 values;
Lines 22-23 check the accuracy;
Lines 24, 205 plot the predictions;
Lines 31-33 additional data QC views
Observed mean radius (horizontal)
vs mean concavity.
Predicted mean radius (horizontal)
vs mean concavity.
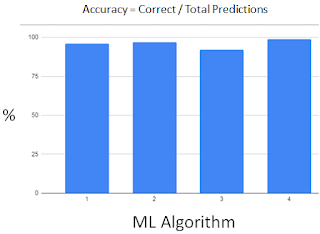
We have tried
several types of classification ML algorithms (ALG):
1 Logistic Regression 95.8%
2 SVM 97.2%
3 Naive Bayes 91.6%
(worst)
4 Random Forest 98.6%
(best)
ALG 1 & 2 yield
similar accuracy,
All ALG yield
acceptable >90% accuracy.
We can make the
confusion matrix
from sklearn.metrics
import confusion_matrix
cm =
confusion_matrix(test,predict)
sns.heatmap(cm,annot=True)
We can view it
as the 2x2 table
|
88(87) |
1(3) |
|
2(3) |
52(50) |
Then we can compute the key
metrics:
P=0.98 (0.96)
R=0.88 (0.96)
Hence,
F1 = 0.92 (0.96) = 0.94 +/-
0.02.
We can see
that our model is working very efficiently and accurately in classifying
whether the BC is of malignant type or benign type. In addition to scikit-learn,
we have visualized the data using pandas and matplotlib libraries.
Technology
AWS now
provides a robust, cloud-based service — Amazon SageMaker — so that developers
of all skill levels can use ML technology. SageMaker API enables developers to create, train, and deploy ML models into a production-ready hosted environment.
The GCP AutoML Tables is another available supervised ML service. It requires example data to train your model by implementing the standard ML ETL pipeline in Figure 4:
1. Gather
your data: Determine the data you need for training and testing
your model based on the outcome you want to achieve
2. Prepare your data:
Make sure your data is properly formatted before and after data import
3. Train: Set parameters
and build your model
4.
Evaluate: Review model metrics
5. Test: Try your model
on test data
6. Deploy and predict:
Make your model available to use.
Here, models can have both numerical and categorical
features.
Finally,
MS Azure ML Studio breaks down ML into five algorithm groups:
- Two-Class (or Binary) Classification
- Multi- Class Classification
- Clustering
- Anomaly Detection
- Regression
In the BCD study the focus is on the Azure ML
Two-Class (or Binary) and Multi-Class Classification algorithms.
References
[1] https://www.healthline.com/health/breast-cancer
[2] https://www.cancer.org/cancer/breast-cancer
[3] https://www.nature.com/articles/s41467-020-17419-7
[4] https://www.neuraldesigner.com/solutions/medical-diagnosis
[5] https://fayrix.com/blog/ten-use-cases-of-machine-learning-for-medical-diagnosis
[6] https://scikit-learn.org/stable/
[7] https://www.anaconda.com/use-cases
[8] https://towardsdatascience.com/best-python-libraries-for-machine-learning-and-deep-learning
[9] https://en.wikipedia.org/wiki/Reinforcement_learning
[10] https://www.mathworks.com/learn/tutorials/reinforcement-learning-onramp.html
[11] https://aws.amazon.com/machine-learning/
[12] https://azure.microsoft.com/en-us/services/machine-learning/
[13] https://cloud.google.com/ai-platform/docs/technical-overview
[14] https://www.geeksforgeeks.org/support-vector-machine-algorithm/
[15] https://www.kaggle.com/uciml/breast-cancer-wisconsin-data
[16] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8612371/
[17] https://towardsdev.com/build-cancer-cell-classification-using-python-scikit-learn-c912af1b61d0
[19] https://techvidvan.com/tutorials/breast-cancer-classification/
[20] https://data-flair.training/blogs/project-in-python-breast-cancer-classification/
[21] https://medium.com/swlh/breast-cancer-classification-using-python-e83719e5f97d
[22] https://www.youtube.com/watch?v=mXy8FE5eFjA
[23] https://www.ijert.org/breast-cancer-classification-using-python-programming-in-machine-learning
[24] https://pyimagesearch.com/2019/02/18/breast-cancer-classification-with-keras-and-deep-learning/










Comments
Post a Comment